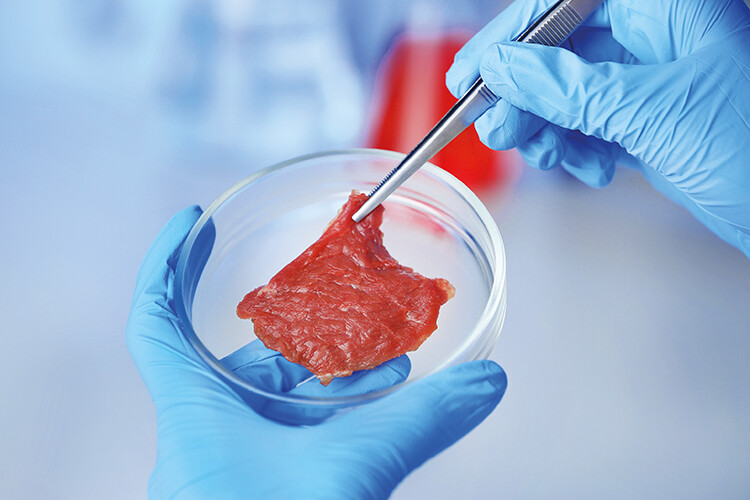
A new breakthrough in lab-grown meat takes agriculture another step away from the farm
By
Meat consumption is on the rise. The world now produces four times the quantity of meat it did 60 years ago; in 2021, the total global production was 350 million tonnes. All that livestock has a heavy environmental footprint and takes up a lot of space – roughly 35 per cent of all habitable land (areas not covered by glaciers or sand) supports the production of meat and dairy.
Consumers looking for environmentally friendly meat face some sizeable obstacles. Intensive animal farming – where a single farm can house more than a million chickens or several thousand cows – is commonly associated with lower standards of animal welfare and the overuse of antibiotics (a key driver of antibiotic resistance worldwide), while organic and free-range animal farming is more resource-intensive and can have higher greenhouse-gas emissions.
Another potential option lies in the business of cellular agriculture, more commonly referred to as lab-grown meat. In 2020, Singapore approved the sale of chicken meat grown from muscle cells – a world first. The USA recently followed suit. Now, researchers from Tufts University in Massachusetts have successfully created ‘immortalised’ bovine muscle cells that can grow quickly and divide hundreds of times, maybe even indefinitely.
David Kaplan, a professor of biomedical engineering and director of the Tufts University Centre for Cellular Agriculture, says the development is critical to the future of lab-grown meat. Normal muscle stem cells, which are sourced from farm-animal biopsies, usually divide only about 50 times before they start to deteriorate. ‘Every time you do a new biopsy, you’re gonna have slightly different cells with slightly different outcomes,’ says Kaplan. ‘You have to start over with the whole [quality assurance and quality control] process. That’s going to be a nightmare, to my mind.’ Having one cell line that every scientist could use would streamline the process, make the end product more affordable and significantly reduce animal use.

To immortalise a cell, researchers genetically modify it by inserting something called a jumping gene (or transposon) into its genome. There’s a large element of trial and error. ‘The idea is to go after the genes that control how often and when the cells will replicate themselves, so that they lose that control and just keep growing,’ says Kaplan. ‘Obviously you want to make sure it’s the right kind of mutation and avoid any sense of tumour formation, which is what we’ve done.’ So far, the study has received a lot of interest from other scientists wanting to use the cells in their own work; Kaplan aims to make them widely available to help move the field of cellular agriculture forward.
Right now, lab-grown meat isn’t the solution to agriculture’s environmental issues. A new, not-yet-peer-reviewed paper from the University of California, Davis, suggests that current production methods could actually be 25 times worse for the climate than regular beef.
When asked whether cellular agriculture could have an environmental edge over farm-reared meat, Kaplan says that the jury is still out. ‘There’s never been food cells grown in a reasonable bioreactor scale to have real data to put into these models. All the stuff you read out there is, to me, fairly suspect, because there are so many assumptions.’ When asked whether it has the potential to solve issues such as food security and land scarcity, however, he says: ‘100 per cent yes – no question about it.’
Although lab-grown meat has been given the green light for consumption in two countries, it will be some time before we see it on our plates. Kaplan also acknowledges that, although it’s safe, not everyone is comfortable with genetically modified food. He’s currently working on another method to immortalise cells that he describes as ‘less GMO-like’, but which he won’t discuss just yet. Nor will he speculate on how long it might take lab-grown meat to appear on supermarket shelves, particularly in Europe, but he says the tools are now there. ‘We’ve already brought the cost down by an order of magnitude. That’s been fairly straightforward and we’ve got the cells. I would argue that the only thing left to solve is scale, and I’m confident it will be solved’.




